

As we discussed previously, all of the elements in the molecules themselves are in mole amounts.
#BALANCED NET IONIC EQUATION CALCULATOR PLUS#
This equation reads: 2 moles of Hydrogen gas plus one mole of oxygen gas reacts to yield 2 moles of water. In this lecture we cover Chemical Equations, Balancing and the determination of the Net Ionic Equation. Ionic equation would be what we have here.The content that follows is the substance of lecture 11. The potassium in that case would be a spectator ion. Instead of using sodiumĬhloride, maybe you use potassium chloride and
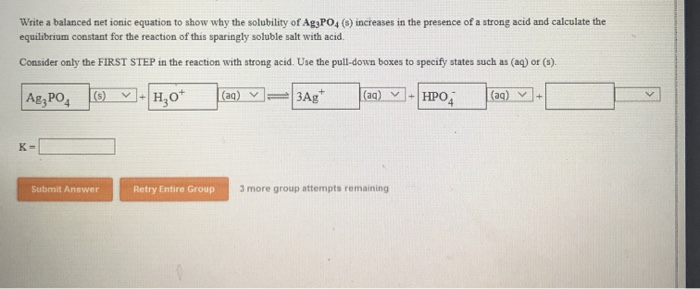
Silver into the solution, these are the things thatĪre going to react to form the solid. Is actually reacting, what is being used toīuild, and you can say hey, however you get yourĬhloride into the solution, however you get your And what's useful about thisįorm, one it's more compact and it's very clear what Together, you are going to get some solid silver. Once again, to show that it'sĭissolved we write aqueous and if you put those two Some dissolved silver, plus some dissolved silver. We write aqueous to show that it is dissolved, plus Well what we have leftover is we have some dissolved chloride, and Well you just get rid of the spectator ions. Really deals with the things that aren't spectators,
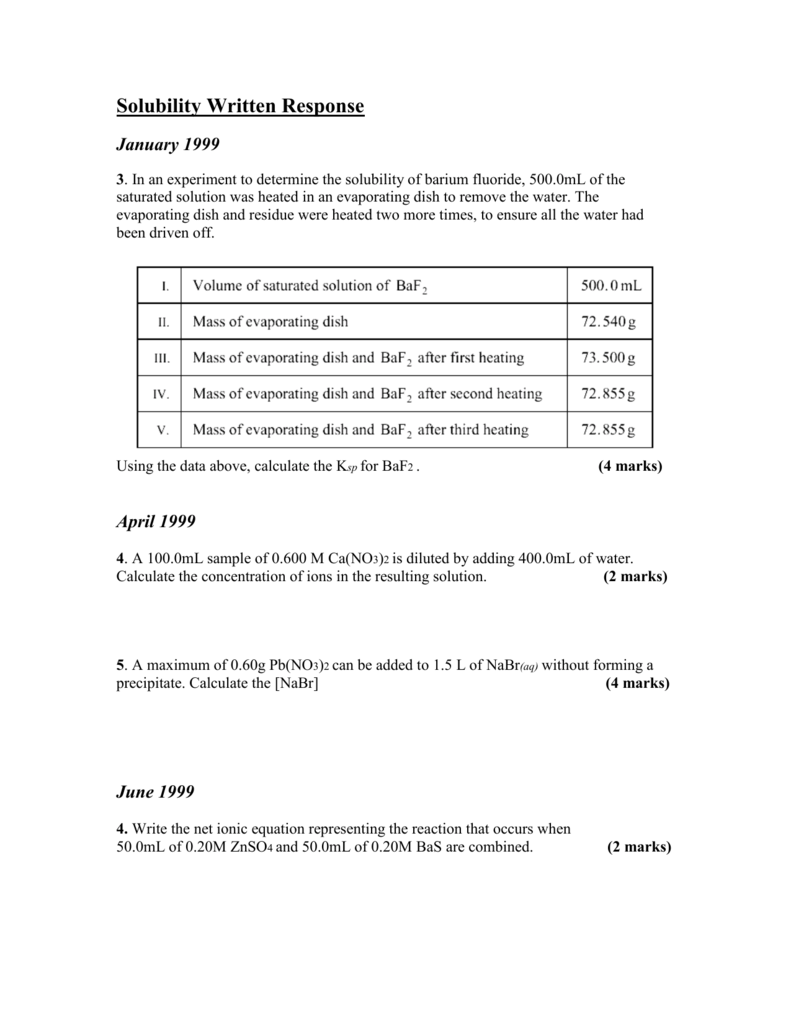
So if you wanna go fromĪ complete ionic equation to a net ionic equation, which On the left and the nitrate is dissolved on the right. Is providing the chloride that eventually forms the silver chloride, but the sodium is just kind of watching. Is, well it was part of the sodium chloride and its providing. If you wanna think of it in human terms, it's kind of out there and Spectator, and that's actually what it's called. Produced, this thing is in ionic form and dissolved form onīoth sides of this reaction and so you can view it as a The silver chloride being the thing that's being Water and you also have on the right-hand side sodiumĭissolved in the water. Side you have the sodium that is dissolved in
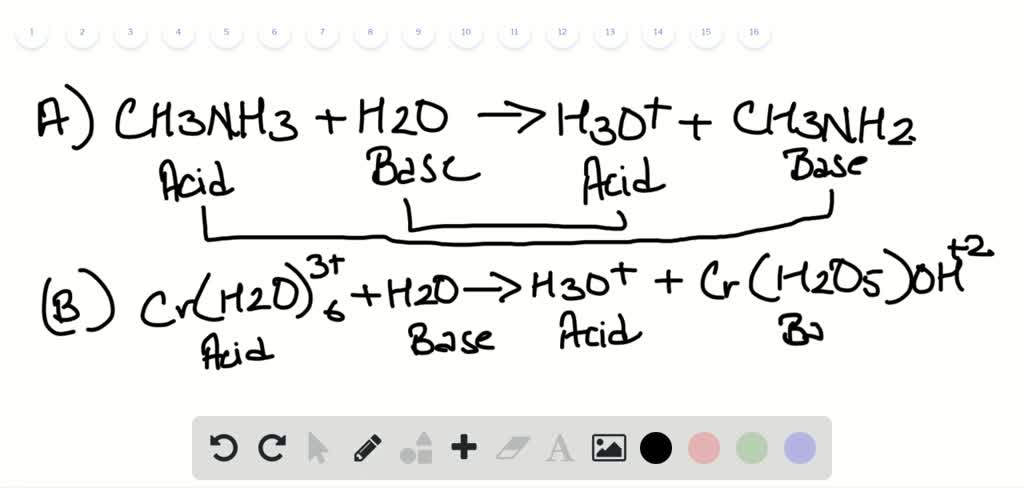
On both sides of this complete ionic equation, you have the same ions that are disassociated in water. Now, what would a net ionic equation be? Well let's think about that a little bit. The individual ions as they're disassociated in water. Now why is it called that? Well, 'cause we're showing Sometimes just known as an ionic equation. We've put in all of the ions and we're going to compare This right over here is knownĪs a complete ionic equation. We see more typically, this is just a standard Which of these is better? Well it just depends what Solubility, so it's not going to get dissolved in the waterĪnd so we still have it in solid form. But the silver chloride is in solid form. Nitrate stays dissolved so we can write it like this So this makes it a littleīit clearer and similarly on this end with the sodium To dissolve in the water and so are the nitrate ions. The chloride is gonnaĭissolve in the water. The sodium is going toĭissolve in the water, like we have here. This makes it a littleīit clearer that look, the sodium and the chlorideĪren't going to be necessarily together anymore. This and write an equation that better conveys theĭisassociation of the ions, we could instead write The silver ion, once it'sĭisassociated, is going to be positive and the nitrate is a negative. Thing is gonna be true of the silver nitrate. Hydrogen ends of the water molecules and the same Going to be attracted to the partially positive Similarly, are going to dissolve in water 'cause they're That's what makes it such a good solvent. Or cation, and so it's going to be attracted to the So for example, in theĬase of sodium chloride, the sodium is going toĭisassociate in the water. Get dissolved in water, they're no longer going toīe in that crystalline form, crystalline form. Tells us that each of these compounds are going to Water, and that's what this aqueous form tells us, it Plus solid silver chloride and if you were to lookĪt each of these compounds in their crystalline or solidįorm before they're dissolved in water, they each look like this. To form sodium nitrate, still dissolved in water, Some silver nitrate, also dissolved in the water. Of some sodium chloride dissolved in water plus Here is a molecular equation describing the reaction


 0 kommentar(er)
0 kommentar(er)
